Silylzinc: Our Reagent
Solid Silylzinc reagents
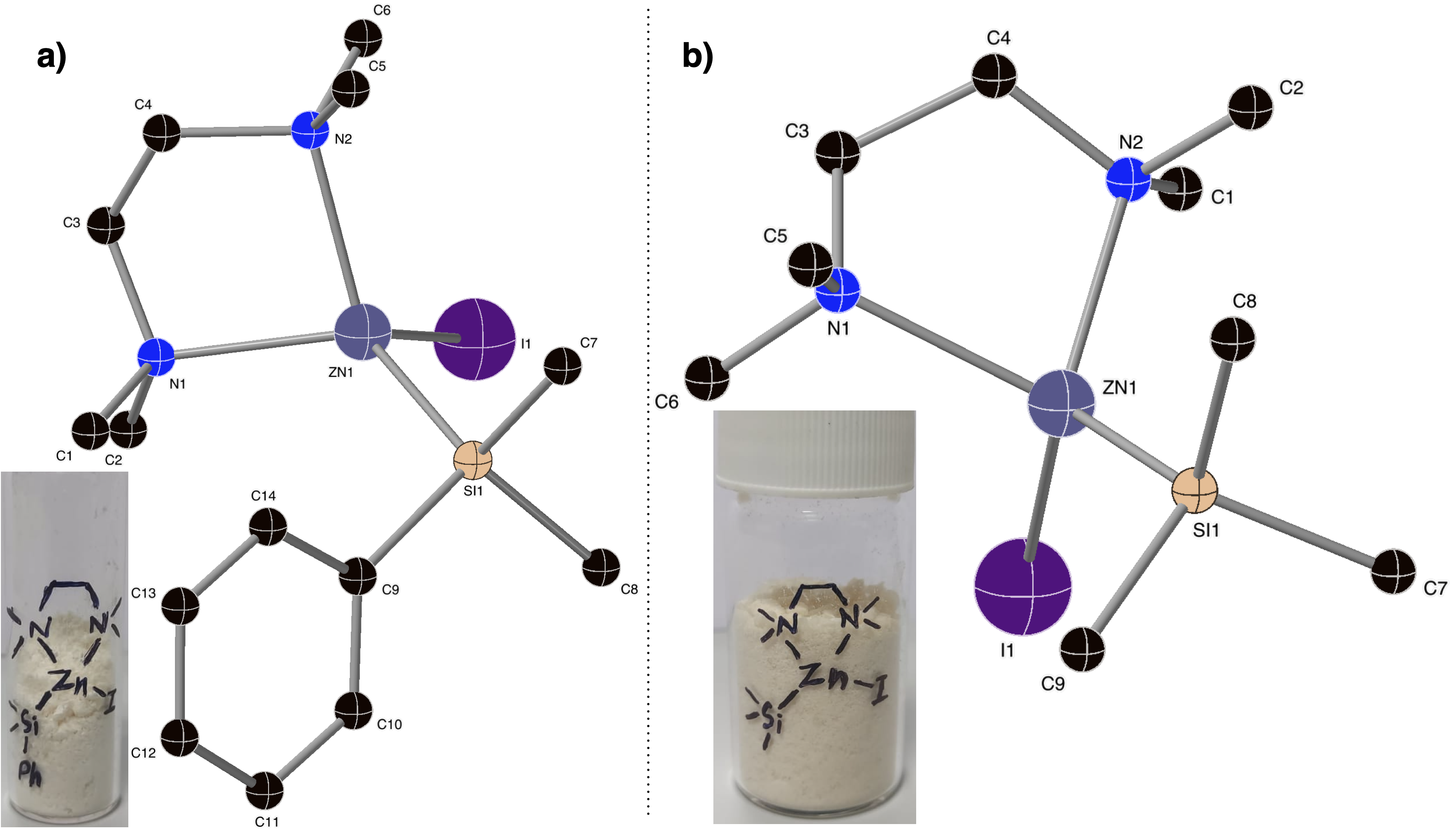
We developed novel silylzinc reagents for the first time. The direct synthesis of PhMe2SiZnI and Me3SiZnI reagents by employing a coordinating TMEDA ligand was achieved. We have also obtained single crystal XRD structures. Importantly, they can be obtained as solids and stored for longer periods at 4 C. Silylzinc reagentsare notoriously difficult to synthesize because they are obtained by a pyrophoric reaction of silyllithium, particularly M33SiLi which is itself prepared by the reaction of MeLi and disilane. Furthermore, the dissolved LiCl in silylzinc may have a detrimental effect. A synthetic method that can avoid silyllithium and involves a direct synthesis of silylzinc reagents from silyl halides is arguably the simplest and most economical strategy.
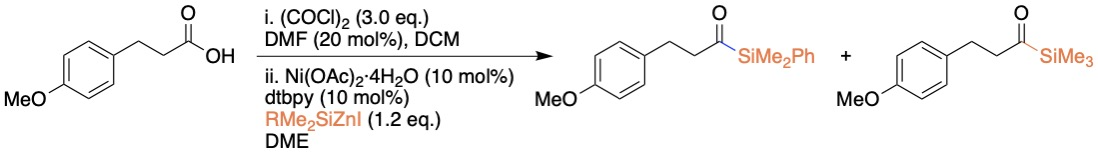
Synthesis of acylsilanes
For the first time, we have developed a method for direct synthesis of silylzinc reagents from silyl iodides and their structures were confirmed by single-crystal XRD. Unlike the use of pyrophoric silyllithium in the synthesis of silylzinc reagents, the current method offers a simplified direct method to access them from silyl halides. The absence of dissolved lithium/magnesium salts in these reagents could be beneficial for various chemical processes. We have also demonstrated the practical synthesis of acylsilanes from unactivated alkyl acid chlorides by nickel, copper and dual catalysis.
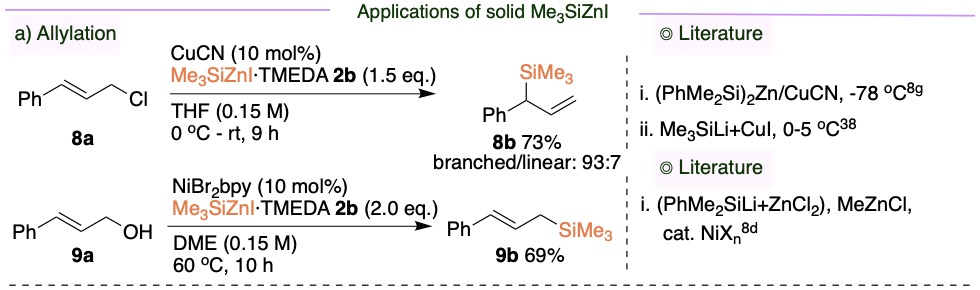
To study the general applicability of this new solid Me3SiZnI, we performed a wide range of organic transformations. Both branched and linear allylsilanes were synthesized through allylation.
The copper-mediated 1,4-addition of Me3SiZnI and PhMe2SiZnI with enones and yielded the β-silyl ketones. We also employed PhMe2SiZnI in a novel Brook-rearrangement/cross-coupling of benzaldehyde and bromoarene.
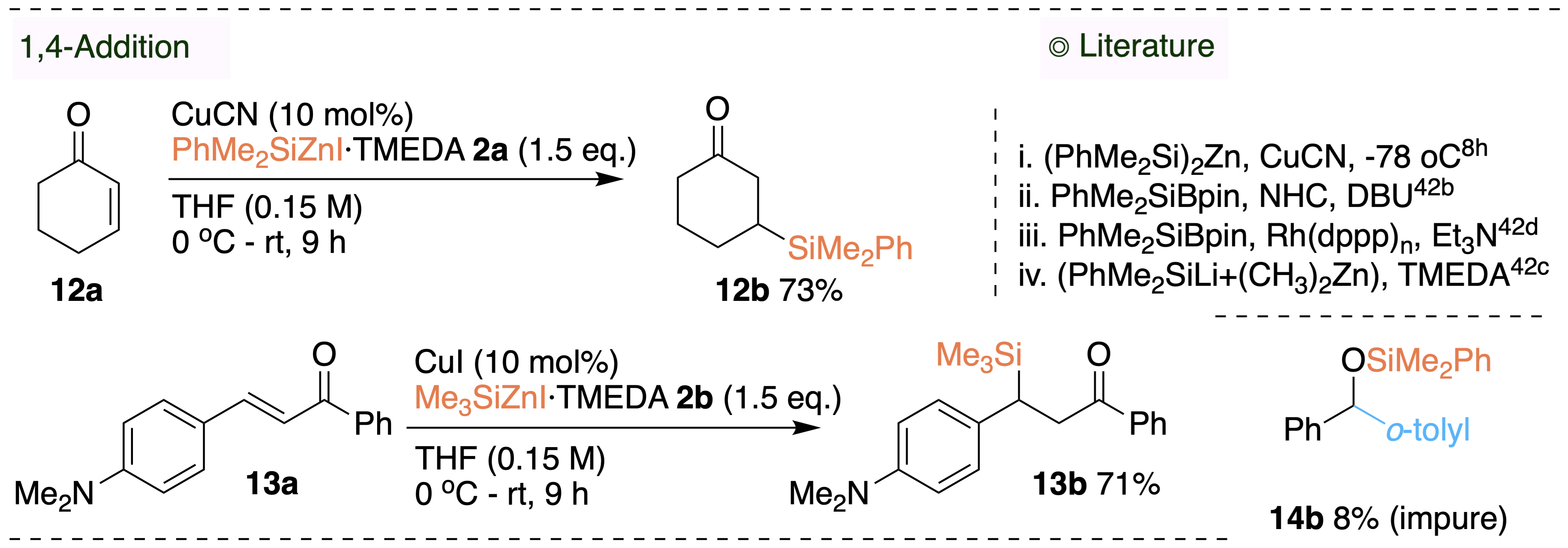
we have demonstrated the efficacy of the novel solid trimethylsilyl zinc reagent in the synthesis of aryl and alkyl trimethylsilanes.
