Cross Electrophile Coupling XEC
Cross Electrophile Coupling XEC
Unlike the class cross coupling reactions, the cross electrophile coupling (XEC) reactions are an attractive alternative since they do not require the highly sensitive organometallic reagents.

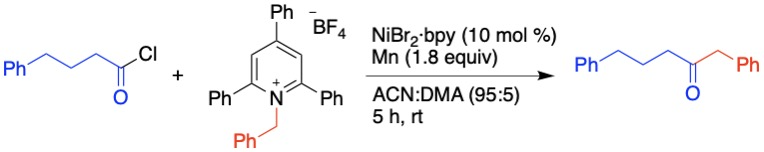
Synthesis of α-Vinyltrialkoxysilanes
Hydrosilylation of alkynes inevitably yields α- and β-isomers of vinyltrialkoxysilanes even with complex ligands and catalysts, limiting its usage in organic synthesis. We report the synthesis of α-vinyltrialkoxysilanes via cross-electrophile C(sp2)–C(sp2) coupling of bromoalkenes.
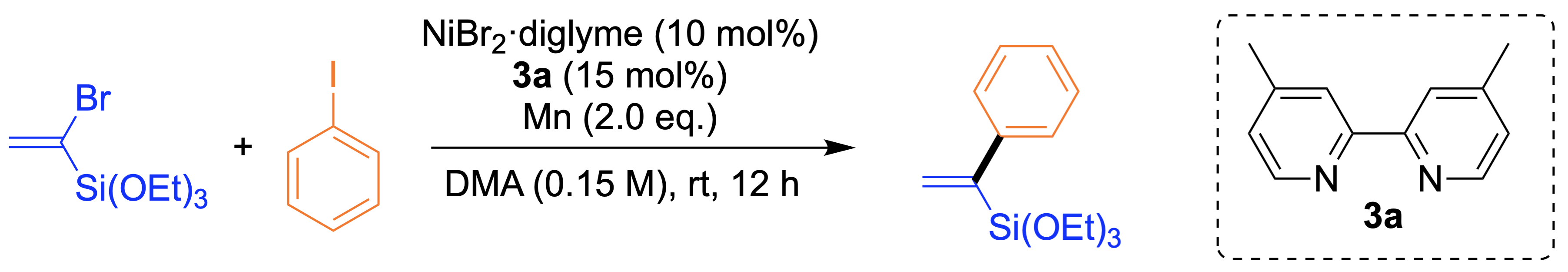
Synthesis of ketones from primary amines
The electrophile−electrophile cross-coupling of carboxylic acid derivatives and alkylpyridinium salts via C−N bond cleavage is developed. Besides acid chlorides, carboxylic acids were also employed as acylating agents, which enabled us to incorporate acid-sensitive functional groups such as MOM, BOC, and acetal.