Photoredox Catalysis
C-H Activation
We utilized dual metallaphotoredox catalysis to achieve the challenging C-H activation and the subsequent cross-coupling reactions.
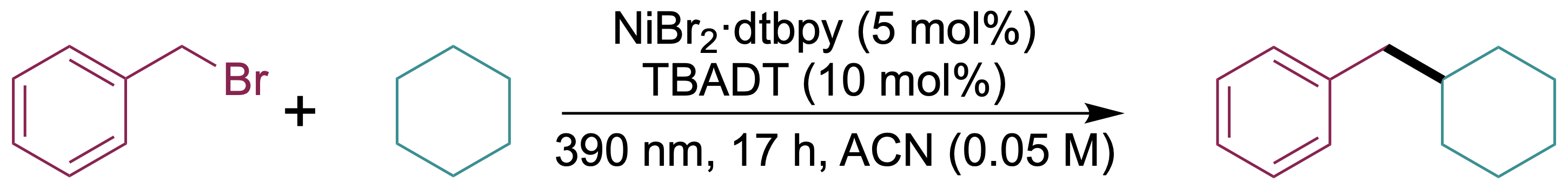
C(sp3)−C(sp3) coupling
we have demonstrated the first C(sp3)−C(sp3) coupling of cycloalkanes with benzyl bromides and primary alkyl iodides via dual TBADT-nickel catalysis. Significantly, TBADT was successfully recovered and recycled up to five consecutive cycles, with no substantial decline in the yield of the coupled product.
Z-alkenes from alkynes
A protocol was developed to facilitate the hydroalkylation of terminal alkynes through the C−H activation of unactivated cyclic/acyclic alkanes. Our cobalt-HAT catalysis achieves the desired Z alkene with excellent regio- and diastereoselectivity via C−H activation.

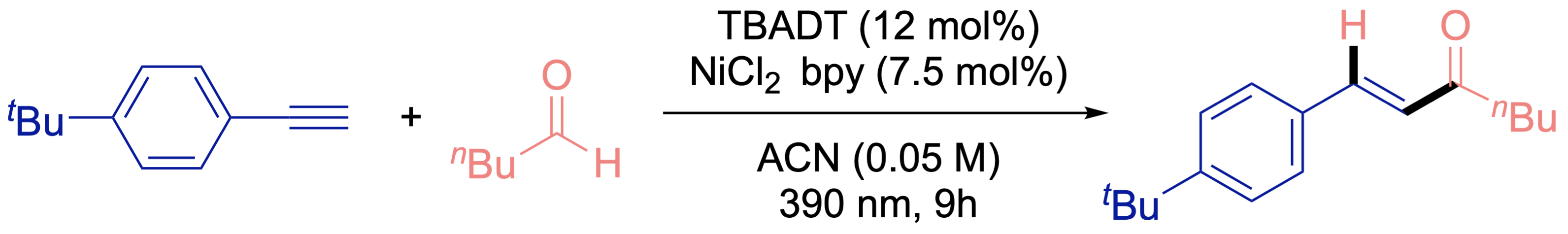
Hydroacylation of Terminal Alkynes
we developed a synergistic nickel− photocatalytic system that allows for the highly regio- and stereoselective hydroxylation of unactivated aldehydes and alkynes in milder conditions without the use of chelating groups.
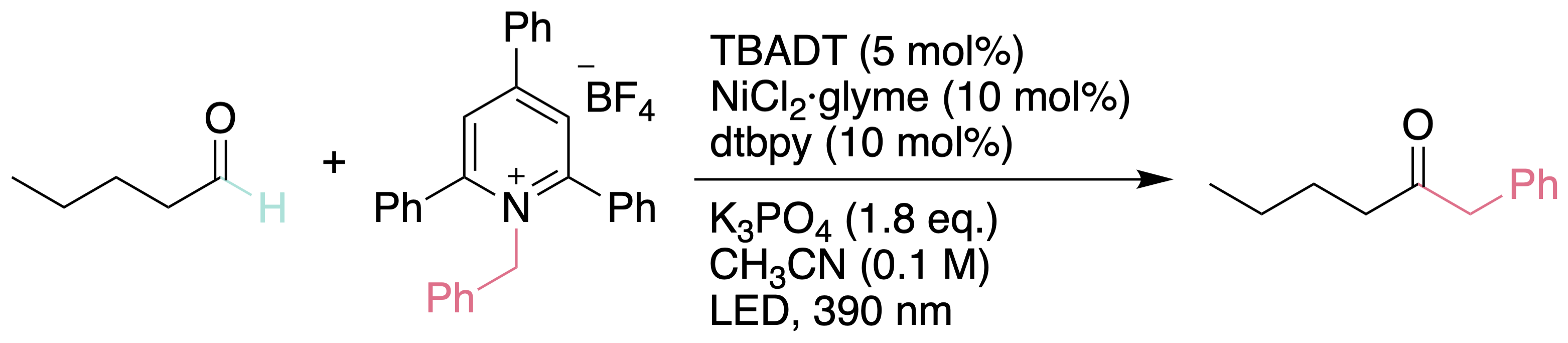
Synthesis of ketones from primary amines
For the first time, we demonstrated a milder and efficient method for the cross-coupling of alkyl and aryl aldehydes with benzylic and allylic pyridinium salts via C−N bond cleavage. Site selectivity was achieved in the presence of various C−H bonds with similar BDEs. α- Amino and α-oxy methylene groups were intact.